Abstract
Background CAR T-cell therapy is a standard of care for patients with r/r LBL and despite promising responses, outcomes can vary depending on the condition of the patient prior to the infusion. Pre-existing comorbidities as scored by the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) (Sorror, et al. Blood. 2005) have been shown to be associated with morbidities and mortality following transplants. The impact of comorbidities on outcomes of CAR T-cell therapy is not well established. To this end, we sought to develop and validate a CAR T-cell therapy-specific comorbidity index (CT-CI) and examine its prognostic value in predicting survival and toxicity outcomes.
Methods Utilizing the cellular therapy registry from the Center for International Blood and Marrow Transplant Research (CIBMTR) adult pts were identified with r/r LBCL receiving commercially available CAR T-cell therapy since FDA approval (2017 and 2018 for axi-cel and tisa-cel, respectively) until December 2020. Each reported individual comorbidity according to the HCT CI was individually assessed on its impact on outcomes after CAR T cell infusion
Pts were randomly assigned to training (67% of pts) and validation (33% of pts) cohorts. In the training set, Cox proportional hazard model was used to examine risk factors associated with OS. A stepwise selection method was used to identify the significant covariates affecting OS with a significance level of 0.01. Pairwise interactions between significant factors were tested. Multivariate analysis (MVA) adjusting for the significant covariates was used to develop a weighted score for each comorbidity based on the magnitude of hazard ratios (HRs) for OS. The CT-CI score (the sum of weighted scores of individual comorbidities) was then tested in the validation cohort.
Results We identified 1916 pts, median age was 63.6 years (range: 18.5-91), with 24.7% (n=473) ≥70 years of age, and 63.9% males (n=1225). A total 13.8% (n=265) and 24.1% (n=461) of pts had double∖triple hit LBCL and transformed lymphoma, respectively. Of the whole cohort 19.5% (n=373) of pts had Karnofsky performance status (KPS) less than 80, while 43.6% (n=836) had an elevated LDH prior to CT. Incidence of all grade CRS was 75.4% (8.5% grade ≥3 and all grade ICANS was 44.4% (19.5% grade ≥3), respectively. 12-month progression free survival (PFS) was 42.2% (95% CI 39.9-44.5) and 12-month OS was 61.6 (95% CI 59.4-63.9). The primary cause of death was disease progression 73.2% (n=617). Incidence of treatment related mortality (TRM) was 4.3% (95% CI 3.4-5.3).
At least 1 comorbidity was recorded for 1321 (68.9%), while 558 (29.1%) had none and 37 (1.9%) did not have reported data. The most commonly reported were history of cardiac disease (11.8%), diabetes requiring non-diet treatment (13.8%), psychiatric disturbance (18.1%), pulmonary disease (moderate- 15.0%, severe- 12.2%) and hepatic disease (mild- 8.2%, moderate∖severe- 2 %).
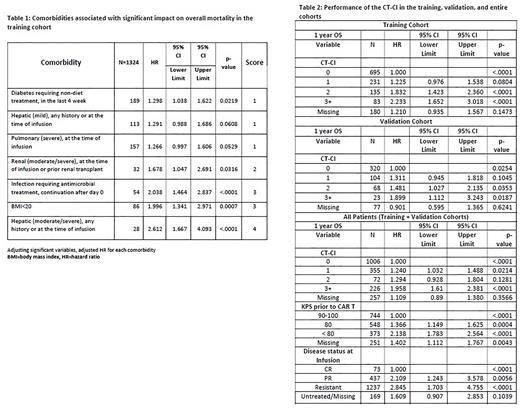
Table 1 shows comorbidities associated with overall mortality on multivariable analysis in the training cohort. Other clinical factors associated with worse OS were lower KPS (<80 vs. 90-100 (HR 1.956, 95%CI 1.577-2.424, p<0.0001, 80-90 vs. 90-100 HR 1.239, 95%CI 1.006-1.527, p=0.0442), and resistant disease or a partial response at time of infusion (resistant disease vs. CR; HR 3.891, 95%CI 1.932-7.836, p<0.0001, PR vs. CR HR 2.789, 95%CI 1.361-5.713, p=0.0051)
Higher CT-CI scores were associated with worse 1-year OS in the training, validation, and entire cohorts (0 vs 1 vs 2 vs ≥3) (Table 2). The C-index was 0.56. While KPS and LDH were associated with toxicities, specific comorbidities and the CT-CI were not associated with development or incidence of CRS or ICANS.
Conclusion Development of a novel CT-CI has shown that comorbidities are predictive of mortality after CAR T-cell therapy and may influence clinical decisions for treatment selection in high risk pt populations. Further analysis will be presented at the meeting.
Disclosures
Awan:BMS: Consultancy; Dava Oncology: Consultancy; Johnson and Johnson: Consultancy; BeiGene: Consultancy; Incyte: Consultancy; Verastem: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy; Pharmacyclics: Consultancy, Research Funding; Gilead Sciences: Consultancy; Kite Pharma: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; MEI Pharma: Consultancy; Merck: Consultancy; Cardinal Health: Consultancy; ADCT Therapeutics: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Caribou Biosciences: Consultancy; Cellecter Bisosciences: Consultancy. Farooq:Kite, a Gilead Company: Honoraria; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Caribou pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Checkmate Pharma: Research Funding. Jain:Incyte: Research Funding; MyeloidTx: Consultancy; BMS: Consultancy; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Kebriaei:Kite: Consultancy; Pfizer: Consultancy; Amgen: Research Funding; Jazz: Consultancy; Ziopharm: Research Funding. Locke:A2: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Education or editorial role; Wugen: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Allogene: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; Clinical Care Options Oncology: Other: Education or editorial role; ASH: Other: Education or editorial role; Imedex: Other: Education or editorial role; BlueBird Bio: Research Funding; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Research Funding; Umoja: Membership on an entity's Board of Directors or advisory committees; Iovance: Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Gerson Lehrman Group: Consultancy; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Education or editorial role; Novartis: Research Funding; Calibr: Membership on an entity's Board of Directors or advisory committees; Emerging Therapy Solutions: Consultancy; Allogene: Membership on an entity's Board of Directors or advisory committees; Cowen: Consultancy; BMS: Research Funding; National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Society for Immunotherapy of Cancer: Other: Education or editorial role. Perales:Orca Bio: Consultancy; Takeda: Honoraria; Medigene: Consultancy; Servier: Consultancy; Bellicum: Honoraria; DSMB: Other; Astellas: Honoraria; Abbvie: Honoraria; Karyopharm: Honoraria; Celgene: Honoraria; Sellas Life Sciences: Consultancy; Cidara Therapeutics: Consultancy; Vor Biopharma: Honoraria; VectivBio AG: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Novartis: Honoraria; Omeros: Consultancy; MorphoSys: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Ramakrishnan Geethakumari:Kite: Consultancy; BMS: Consultancy; Rafael Pharma: Consultancy; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cellectar Biosciences: Membership on an entity's Board of Directors or advisory committees. Shouval:Medexus: Consultancy, Ended employment in the past 24 months; MyBiotics: Consultancy. Shpall:axio: Consultancy; Bayer: Honoraria; adaptimmune: Consultancy; Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; Affimed: Other: License agreement; Navan: Consultancy; Takeda: Patents & Royalties. Turtle:Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno Therapeutics, a BMS Company: Patents & Royalties, Research Funding; Precision Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Caribou Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Arsenal Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Decheng Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Expert Connect: Consultancy; Allogene: Membership on an entity's Board of Directors or advisory committees; Prescient Therapeutics: Membership on an entity's Board of Directors or advisory committees. Pasquini:Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Kite: Research Funding; Janssen: Research Funding. Ahmed:Tessa Therapeutics: Consultancy, Research Funding; Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Merck: Research Funding; Myeloid Therapeutics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Seagen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal